ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 27th May. Click Here for more information.
Source: The post is based on the article “India rejects Johnson & Johnson’s attempt to extend monopoly on lifesaving TB drug” published in The Hindu on 24th March 2023
What is the News?
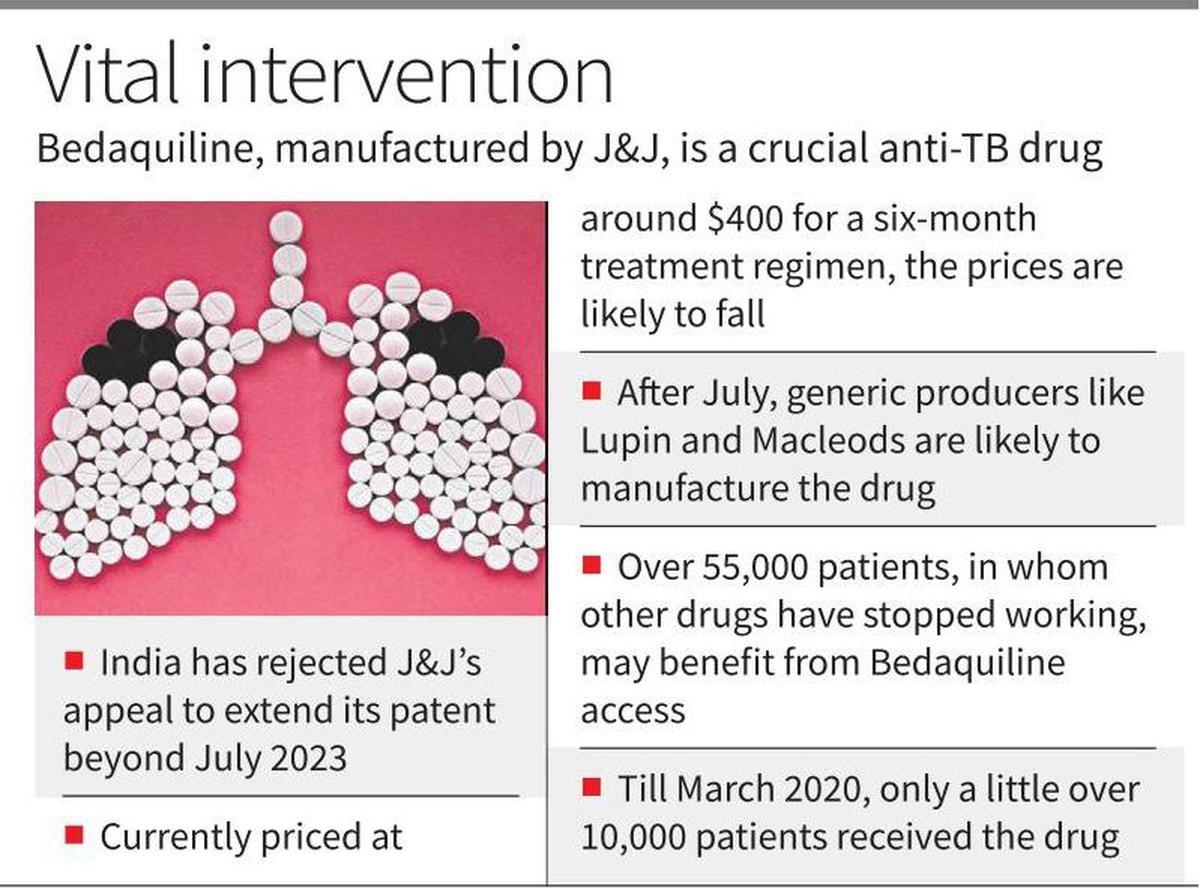
The Indian Patent Office has rejected U.S. pharmaceutical giant Johnson & Johnson’s (J&J) attempt to extend its monopoly on the manufacturing of the anti-tuberculosis drug Bedaquiline in India beyond July 2023.
The patent office invoked Section 3(d) in its judgment as the Indian patent law does not allow the evergreening of patents and prevents pharma majors from extending the patent beyond the stipulated monopoly on the drug.
Note: Since 2007, J&J has indulged in ‘evergreening’ by making multiple claims in its applications for patent extensions.
What is Bedaquiline?
Bedaquiline is used in combination with other medicines to treat tuberculosis patients when the first line of treatment fails to kill the bacteria.
The use of this oral drug has been crucial for tuberculosis treatment in the country. It has lesser-known harmful effects than injectable drugs.
The cost of tuberculosis treatment by bedaquiline is expected to significantly drop due to the rejection of the patent as it would allow Indian manufacturers to manufacture generic medicines.
What is Evergreening?
“Evergreening” is a term used in the context of intellectual property rights, particularly with regard to patents. It refers to the practice of extending the exclusivity period of a patent by making small modifications or improvements to the original invention.
The goal of evergreening is to extend the period during which the original inventor can profit from their invention. This is typically done by filing for new patents that cover variations or modifications of the original invention, even if those modifications are relatively minor.
Critics of evergreening argue that it stifles innovation by preventing other inventors from building on existing technology.They also argue that it drives up the cost of drugs and other patented products, making them less accessible to people who need them.
What is Section 3(d) of the Indian Patent Act?
Section 3(d) of the Indian Patent Act 1970 (as amended in 2005) states that inventions that are mere “discovery” of a “new form” of a “known substance” and do not result in increased efficacy of that substance are not patentable.
This implied that India did not support patents for inventions which were minor modifications and prevented undue monopoly during the extended period of patent protection by the company.




