Concept of Global Warming:
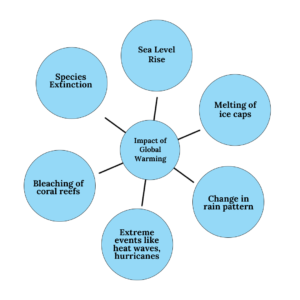
Global Warming is a gradual heating of the earth’s surface, oceans and atmosphere. The increase in temperature is often a result of the Greenhouse Effect caused by increased levels of gases like Carbon Dioxide, CFCs and other pollutants. As per IPCC report, the world is 1.2oC warmer than pre-industrial levels.
Greenhouse Effect involves following steps:
1. High energy short wavelength solar radiation reaches the earth’s atmosphere.
2. About 30% of the sun’s energy is reflected directly back into the space by the atmosphere, clouds and surface of the earth. The rest of the energy is absorbed into the earth’s system.
3. The Earth re-emits energy in the form of infrared radiation back into the atmosphere, at wavelengths longer than incoming solar energy.
4. Greenhouse gases in the atmosphere like Water Vapor, Carbon Dioxide, Methane, Nitrous Oxide, Fluorinated Gases like HFCs and SF6, Black Carbon and Brown Carbon, absorb much of the long wave energy (infrared radiation) emitted from the earth’s surface. Thus, these gases prevent the energy from escaping the earth’s surface, leading to warming of the atmosphere.
Global Warming Potential (GWP) for a gas is a measure of the total energy that a gas absorbs over a period of time, compared to carbon dioxide. Gases with high GWP absorbs more energy than gases with lower GWP, thus contributing more to warming earth.
| GHG CO2 CH4 N2O HFC – 23 HFC – 134a SF6 | GWP for 100 years 1 23 296 12000 1300 22200 |
The term ‘Carbon Fertilization’ is often used in the context of Greenhouse Gas emissions, which means high rate of plant growth due to increased concentration of CO2 in the atmosphere.
Black Carbon:
- It is commonly known as soot, is a solid particle or aerosol produced from incomplete combustion. It is released from biomass burning, cooking with solid fuels, vehicular emissions etc.
- Black Carbon warms the earth by absorbing heat in the atmosphere and reducing albedo when deposited on snow. This is turn accelerates the melting of glaciers. Further, it also disrupts precipitation patterns.
- Black Carbon is a Short-Lived Climate Pollutant (SLCP).
Brown Carbon:
- It is brown smoke released by the combustion of organic matter. It co-exists with Black Carbon when released in the atmosphere.
- Black Carbon is primarily released by high temperature combustion (diesel engines etc.) and Brown Carbon is mainly released by biomass combustion.
Fluorinated Gases:
- HFCs, PFCs, SF6 are emitted through a variety of industrial processes like aluminum and semi-conductor manufacturing, substitution for ozone depleting substances, electrical transmission equipment.
- Fluorinated Gases have much longer lifetime compared to gases like Methane and Nitrous Oxide in the following order: SF6/PFCs>HFCs>Nitrous Oxide>CO2>CH4
Nitrous Oxide:
- It is naturally present in the atmosphere as a part of earth’s Nitrogen Cycle.
- Natural sources include bacteria breaking down nitrogen in soils while human induced factors include agriculture, transportation and industry (fertilizers industry and in the production of adipic acid used to make fibers like nylon).
- Poultry industry also releases active nitrogen compounds into atmosphere
Methane:
- It is emitted from natural sources like wetlands as well as human activities like agriculture (anaerobic conditions associated with rice cultivation), livestock, industry and wastes from home.
- It is much more harmful GHG than CO2
Concept of Ocean Acidification:
Ocean Acidification is called the “evil twin of global warming” and “the other CO2 problem”. It refers to the ongoing decrease in the pH of the earth’s oceans, caused by the uptake of CO2 from the atmosphere.
Other contributors to ocean acidification are:
- Acid Rain can lower the pH of the oceans.
- Eutrophication leads to large plankton blooms and when these blooms collapse and sink to the bottom of the oceans, the subsequent respiration of bacteria decomposing the algae leads to a decrease in sea water oxygen and increase in CO2 (a decline in pH).
Impacts of ocean acidification are as follows:
- Increase in acidity depresses metabolic rates and immune response in some organisms.
- Survival of some animals having phytoplanktonic larvae will be adversely affected.
- Increased acidity leads to coral bleaching.
- As the pH of the ocean declines, the concentration of Carbonate ions decreases. This makes it more difficult for marine calcifying organisms like corals and some plankton to form biogenic calcium carbonate.
- Commercial fishing is threatened because acidification harms calcifying organisms which form the base of marine food webs.
- Cloud Seeding and formation of clouds will also be adversely affected.
Artificial Cloud Seeding is the process of spreading either dry or more commonly, silver iodide aerosols, into the upper parts of the clouds to stimulate the precipitation process and form rain.
Concept of Ozone Depletion:
Ozone is a natural gas and an allotrope of oxygen consisting of three atoms of oxygen bound together. Ozone is found in two different layers of atmosphere; Ozone in the troposphere is bad as it leads to smog formation while stratospheric ozone is good because it protects the life on earth from the harmful UV rays of the sun.
Impacts of Ozone Depletion:
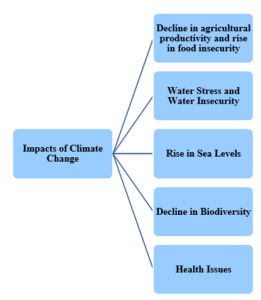
- Due to Ozone depletion, more and more UV rays are able to reach Earth. These harmful rays can cause cataracts, skin cancer and impaired immune systems.
- UV rays can also damage sensitive crops, such as soyabeans and reduce crop yields. This in turn can impact food supplies.
- Marine phytoplanktons, which are at the base of the ocean food chain, are also under stress from UV radiation and their productivity is declining.
Sources of Ozone depletion include:
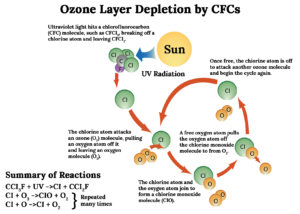
- Chlorofluorocarbons (CFCs): These are used as refrigerants, propellants in aerosol sprays, foaming agents in plastic manufacturing, fire extinguisher agents, solvents for cleaning electronic components, for freezing foods etc.
- Nitrogen Oxides: The source of Nitrogen Oxides are mainly explosions of thermonuclear weapons, industrial emissions and agricultural fertilizers.
Other substances like Bromine containing compounds called halons (used in fire extinguisher), HBFCs (Hydrobromofluorocarbons) and Methyl Bromide (used as pesticide/fumigant) also destroy Ozone. Each Bromine atom destroys hundred times more Ozone molecules than what a Chlorine atom does.
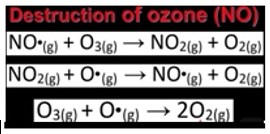
- Polar Stratospheric Clouds (PSCs): These are nacreous clouds containing water, nitric acid and/or sulphuric acid. They are formed mainly during the event of polar vortex in winter and are more intense at the South Pole/Antarctica. PSCs are a source of Ozone depletion because they support chemical reactions that produce active Chlorine which catalyses Ozone depletion.
| Ozone depletion is more over Antarctica than Arctic because the very low winter temperatures in the Antarctica Stratosphere cause Polar Stratospheric Clouds (PSCs) to form. |