ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 19 April. Click Here for more information.
What is the News?
Drugs Controller General of India (DCGI) has approved the Phase II/III clinical trials of the CORBEVAX vaccine in children aged above 5 years. It has also received the nod for conducting Phase III trials in adults.
About CORBEVAX Vaccine:
CORBEVAX is an indigenously produced COVID-19 vaccine candidate developed by the Indian pharmaceutical firm Biological E. Limited (BioE).
It is a “recombinant protein sub-unit” vaccine which means it is made up of a specific part of SARS-CoV-2 — the spike protein on the virus’s surface.
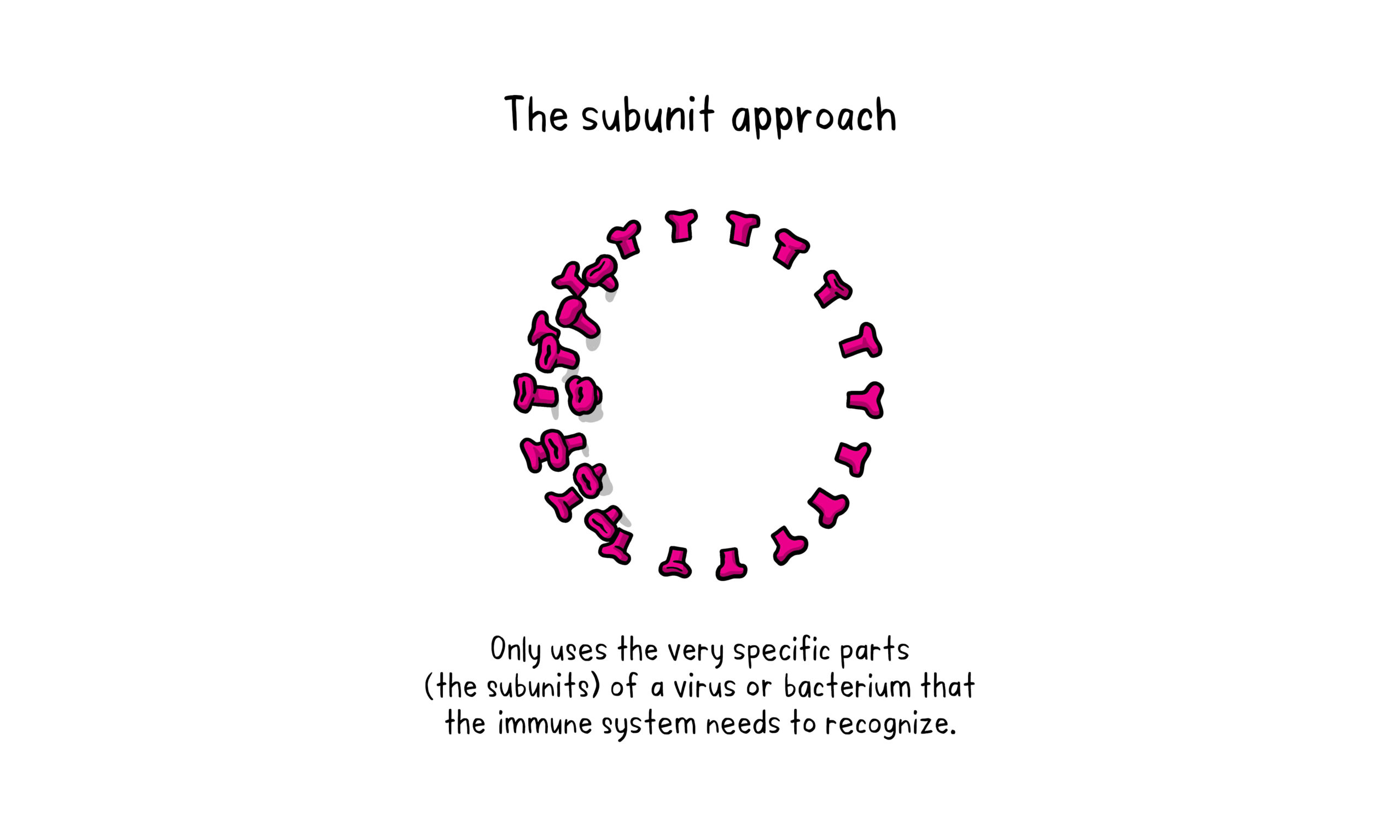
About Subunit Vaccine:
Source: WHO
A subunit vaccine is one that only uses the very specific parts (the subunits) of a virus or bacterium that the immune system needs to recognize.
It doesn’t contain the whole microbe or uses a safe virus as a vector. The subunits may be proteins or sugars.
Source: This post is based on the article “DCGI approves clinical trials of Biological E. Covid-19 vaccine CORBEVAX ” published in PIB on 4th September 2021.