ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 19 April. Click Here for more information.
Contents
- 1 Introduction
- 2 About Molnupiravir and its Emergency Use Authorisation
- 3 What are the concerns associated with Molnupiravir?
- 4 What is the ‘Emergency Use Authorisation’ (EUA)?
- 5 What are the drugs that have received Emergency Use Authorisation in the treatment of Covid-19?
- 6 What is ICMR’s national task force on covid management?
- 7 How is the difference of opinion between ICMR and CDSCO hurting India’s Covid response?
- 8 What are the other challenges in drug regulation?
- 9 What should be done?
| For 7PM Editorial Archives click HERE → |
Introduction
On January 14, 2022 about 33 leading public health experts shot off an open letter to the Central and State Ministries of Health, and to the Indian Medical Association. The letter raised several issues, including the fact that expensive diagnostics and medications with limited evidence were being promoted in India under the Emergency Use Authorisation of drugs to manage the pandemic.
They urge the state “to intervene to stop the use of medications and diagnostics that are inappropriate for the clinical management of Covid-19.” They urged the government to discourage the use of “alternative therapies, potions, antibodies, ‘cocktails’, and drugs like Molnupiravir, which are expected to be widely abused”.
About Molnupiravir and its Emergency Use Authorisation
Molnupiravir was initially developed to treat influenza. Now it is being repurposed as an oral antiviral candidate to treat Covid-19 patients. It works by introducing errors into the SARS-CoV-2 virus’ genetic code, which prevents the virus from further replicating in the immune system.
Molnupiravir received emergency use approval from India’s drug regulator, the Central Drugs Standard Control Organization (CDSCO), as a treatment for mild and moderate Covid-19 patients in the last week of December 2021. The CDSCO asked the pharma companies to communicate the risks and side effects of the drug to physicians. Eight Indian generic companies launched the drug as the first line of treatment.
Few days before, the director-general of the Indian Council of Medical Research (ICMR) raised an alarm over the use of molnupiravir. He mentions, “the known and unknown risks of the drug outweigh its benefits.”
| Must Read: What is Molnupiravir? |
What are the concerns associated with Molnupiravir?
While approving the Molnupiravir, the United States Food and Drug Administration (USFDA) said that 1. Molnupiravir is not authorized for use in patients younger than 18 years of age because it may affect bone and cartilage growth. 2. The drug is not authorized for the pre-exposure or post-exposure prevention of Covid-19 or for initiation of treatment in patients hospitalized due to Covid-19. 3. Additionally, the drug is also not recommended to be used among pregnant women, as in lab studies the drug was shown to cause fetal harm.
Apart from that, the drug also has the following concerns.
Low Effectiveness: The drug was found to be only 30% effective in reducing risk from hospitalization or death in trials, much lower than earlier indications.
Worries over Mechanism of the Drug: The drug works by incorporating itself into the RNA of the virus, inducing mutations with the objective of hampering replication.
But this carries the risk of introducing mutations that can make the virus stronger and more dangerous. There is also a bigger risk of the drug is of creating mutations in the human DNA itself.
What is the ‘Emergency Use Authorisation’ (EUA)?
The Drug and Cosmetics Act, 1940 establishes regulatory control over the import, manufacture, distribution, and sale of drugs and cosmetics in India. The Act also established the Central Drugs Standard Control Organization (CDSCO) for discharging functions assigned under the Drugs and Cosmetics Act. CDSCO is headed by the Drugs Controller General of India (DCGI).
| Read more: Medical devices now under Drugs and Cosmetics Act |
In a pandemic situation, it may not be possible to have all the evidence that a drug regulator would normally require for approving a drug, vaccine, device or test. When there is a declared emergency, the regulator (DCGI), can take a call whether it is worth releasing a drug or vaccine that is not fully tested for efficacy and safety.
Such emergency use authorisation to a medical product will make it widely available for use.
| Must read: Drug Regulations in India – Explained, pointwise |
What are the drugs that have received Emergency Use Authorisation in the treatment of Covid-19?
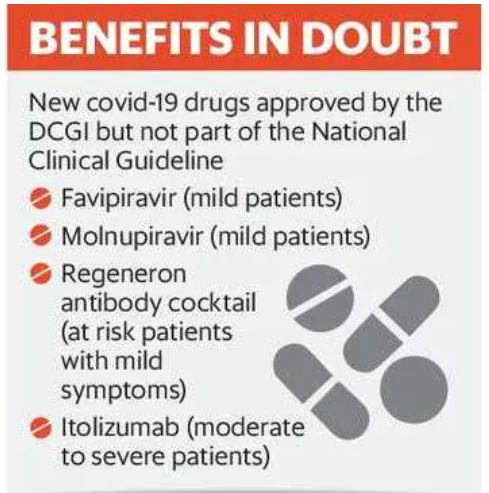
In the last two years, the CDSCO has issued Emergency Use Authorisation to many drugs with limited evidence in improving the condition of Covid-19 patients.
For instance, 1. Itolizumab: It is a monoclonal antibody that was launched at a price of ₹32,000 for four vials. It was approved in July 2020 based on a clinical trial done on 30 patients. 2. Favipiravir: It is one of the commonly used Covid-19 drugs. It was approved in 2020. According to a study published in November 2020, in the medical journal Elsevier, favipiravir did not have a statistically significant difference in a patient’s recovery.
3. An antibody cocktail drug by US drugmaker Regeneron Pharmaceuticals, approved in India in May 2021.

According to Regeneron, it is not effective against the Omicron variant. The USFDA, too, has revised its guideline and advised against the use of the drug as it is ineffective in the Omicron wave. Despite emerging evidence, the DCGI’s office has not issued any clarification on the use of the drug.
What is ICMR’s national task force on covid management?
A National Task Force (NTF) for COVID-19 has been constituted by ICMR. The task force will initiate research studies and identify priorities for clinical research, diagnostics and biomarkers, epidemiology and surveillance, vaccines and drug development to combat the coronavirus.
The NTF consists of 21 members, including technical/domain experts from the government and outside the government. So far, the Task Force has held over 20 meetings and has systematically contributed towards the scientific and technical response to the pandemic.
Some of the above-mentioned drugs which receive EUA have not been recommended by NTF due to a lack of evidence on clinical benefit. The task force’s current guidelines prescribe a limited set of drugs for the treatment of Covid-19. They include anti-asthma drug budesonide for mild patients, remdesevir for moderately ill patients, and tocilizumab in extreme cases.
| Must read: Anti-Covid pill Molnupiravir: Approved, not recommended |
How is the difference of opinion between ICMR and CDSCO hurting India’s Covid response?
According to the experts, the differing views between the drugs controller and ICMR’s clinical guidelines are now hurting India’s Covid-19 response, jeopardizing public health. The lack of coordination between ICMR and CDSCO is leading to irrational drug use, confusion among the medical community and additional treatment costs to patients.
Many drugs are now being prescribed by private hospitals. According to the experts, the average cost of hospitalization due to Covid-19 in a private hospital can range anywhere between ₹50,000 and ₹2 lakh.
| Read more: INDIAN PHARMACEUTICAL SECTOR CHALLENGES AND REFORMS |
What are the other challenges in drug regulation?
Challenges in monitoring: Once a drug is approved by the regulator, it is hard to monitor how the drug is being administered.
Opaque functioning of DCGI: There is little information on the drug and vaccine approval processes for Covid-19—on the evidence that was considered. The drugs controller is guided by a group known as the ‘subject expert committee’. In the last two years, there has been little or no disclosure on the members approving Covid-19 drugs and vaccines. This opaque decision-making hurts public health, especially since most Indians pay out of their pockets.
Systemic challenges in Drug regulation: Indian State and Drug Regulators often have to deal with problems like poor training, antiquated record-keeping systems, understaffing, pressure from the pharmaceutical industry, etc. This led them ill-equipped to enforce recalls and root cause analysis.
What should be done?
First, India needs rational use of drugs in the private and public sectors, especially during the pandemic.
Second, there cannot be two separate clinical guidelines. It is absolutely necessary that the ICMR and the drug controller should work together while approving critical Covid-19 drugs. To achieve it, a synchronized playbook—where the ICMR and the drugs controller work together—should be put in place.
Third, the CDSCO should follow the USFDA in terms of transparency: USFDA is considered to be one of the most stringent regulators in the world. Before a drug is taken up for approval, companies in the US have to make a public presentation to an independent panel of experts on the product. This presentation is open to the public.
Once a drug is approved for emergency use, the USFDA releases a detailed statement on the uses of the drug, the side effects, and its effect on various population groups. This is backed by several scientific papers that support the rationale behind the approval. Such best practices should be adopted in India.
Fourth, address the systemic issues in Drug regulation: There is a need to provide adequate training to the staff. Also, the vacancies should be filled immediately so that they are not overburdened.
Covid in 2022 is much different from what it was in 2020. Two years ago, physicians had no idea what treatments work and hence there was some rationale in prescribing some of these drugs. But now, there is much clearer evidence of what works and what doesn’t. Hence, It is no longer acceptable to prescribe drugs only because of a lack of coordination between DCGI and NTF.




