ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 27th May. Click Here for more information.
Source: The post is based on the article “Bharat Biotech’s intra-nasal COVID vaccine gets emergency use approval” published in PIB on 7th September 2022
What is the News?
Bharat Biotech International Limited (BBIL) gas announced that iNCOVACC (BBV154) vaccine has received approval under Restricted Use in Emergency Situation for ages 18 and above.
What is iNCOVACC?
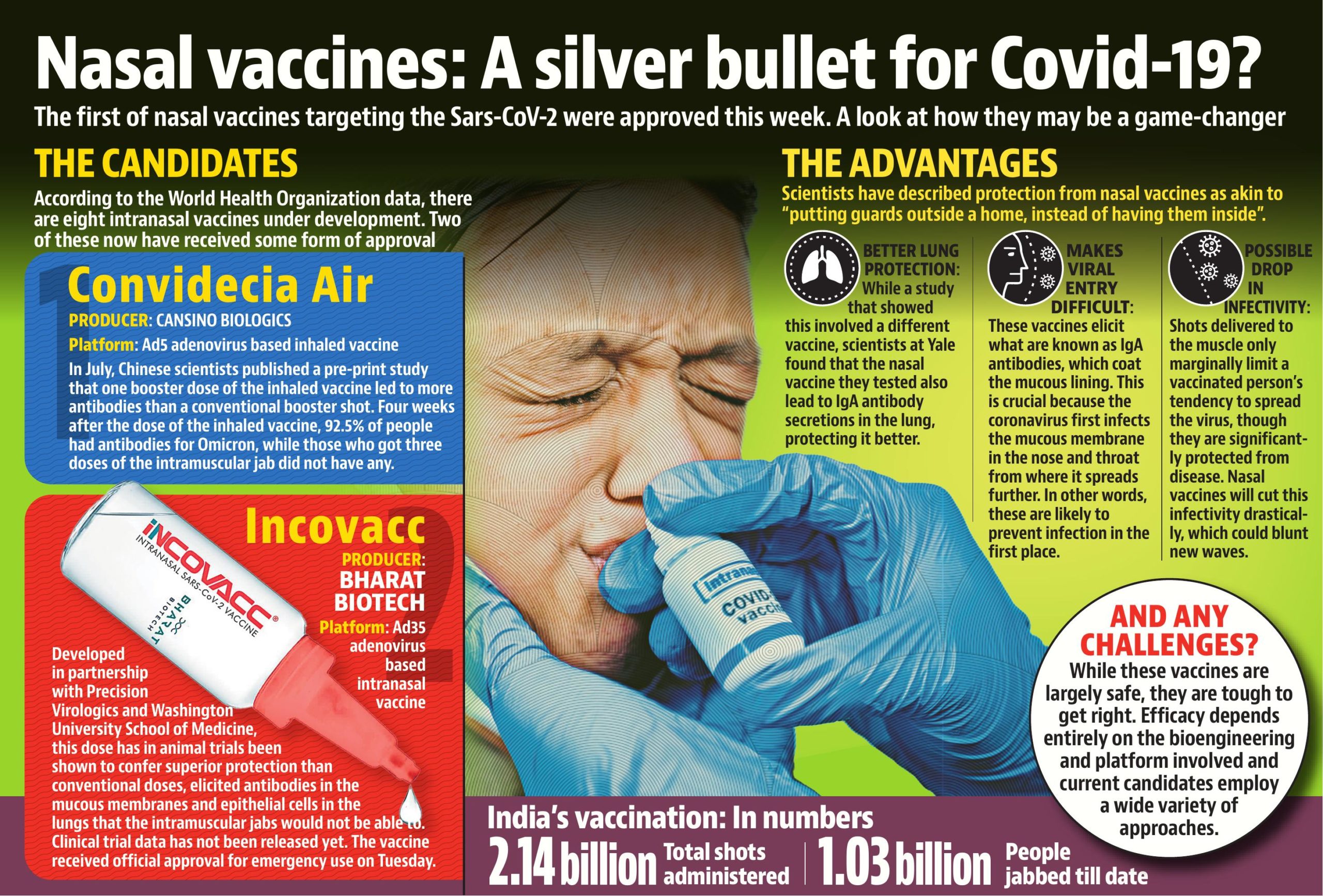
It is an intra-nasal Covid-19 vaccine.
Developed by: Bharat Biotech International Limited (BBIL)
Working: It is a recombinant replication-deficient adenovirus vectored vaccine with a prefusion stabilized spike protein. It uses a modified chimpanzee adenovirus which cannot replicate in the body to carry Covid spike protein to induce immunity.
Usage: This vaccine will be used for primary immunization against COVID-19 in the 18+ age group for restricted use in emergency situations.
Storage: The vaccine is stable between two and eight degrees Celsius for easy storage and distribution.
What are the benefits of iNCOVACC?
1) Likely to block both infection and transmission of COVID-19, 2) Non-invasive and needle-free, 3) Easy to administer as it does not require trained healthcare workers, 4) Eliminates needle-associated risks (injuries and infections), 5) High compliance — ideally suits children and adults and 6) Scalable manufacturing — able to meet global demand.




