ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 27th May. Click Here for more information.
Contents
Source: The post is based on the article “CAR T-cell therapy: the next step towards a holistic treatment of cancer” published in Indian Express on 7th February 2023.
What is the News?
The three major forms of treatment for any cancer are surgery (removing the cancer), radiotherapy (delivering ionising radiation to the tumour), and systemic therapy (administering medicines that act on the tumour).
A new development in the treatment of cancer currently holding the attention of many researchers worldwide is CAR T-cell therapy.
What are CAR T-cells?
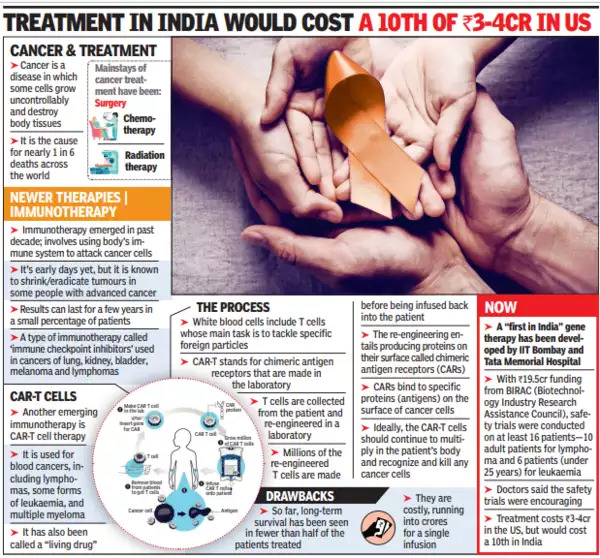
Chimeric antigen receptor (CAR) T-cell therapies represent a quantum leap in the sophistication of cancer treatment.
Unlike chemotherapy or immunotherapy, which require mass-produced injectable or oral medication, CAR T-cell therapies use a patient’s own cells. Hence, they’re called ‘living drugs’.
How does CAR T-Cells therapy work?
In CAR T-cell therapy, the patient’s blood is drawn to harvest T-cells which are immune cells that play a major role in destroying tumour cells.
Researchers then modify these cells in the laboratory so that they express specific proteins on their surface, known as chimeric antigen receptors (CAR). They have an affinity for proteins on the surface of tumour cells.
These modified cells are then infused back into the patient’s bloodstream after conditioning them to multiply more effectively.
This modification in the cellular structure allows CAR T-cells to effectively bind to the tumour and destroy it. The final step in the tumour’s destruction involves its clearance by the patient’s immune system.
Where is CAR T-Cell therapy used?
CAR T-cell therapy has been approved for leukaemias (cancers arising from the cells that produce white blood cells) and lymphomas (arising from the lymphatic system).
It is also used among patients with cancers that have returned after an initially successful treatment or who haven’t responded to previous combinations of chemotherapy or immunotherapy.
How widespread CAR T-cell therapy is used?
The complexity of preparing CAR T-cells has been a major barrier to their use. The first clinical trial showing they were effective was published almost a decade ago.
The first indigenously developed therapy in India was successfully performed only in 2022.
Moreover, trials are underway in India, with companies looking to indigenously manufacture CAR T-cells at a fraction of the cost. The preliminary results have been encouraging.




