ForumIAS announcing GS Foundation Program for UPSC CSE 2025-26 from 19 April. Click Here for more information.
ForumIAS Answer Writing Focus Group (AWFG) for Mains 2024 commencing from 24th June 2024. The Entrance Test for the program will be held on 28th April 2024 at 9 AM. To know more about the program visit: https://forumias.com/blog/awfg2024
Source: The post is based on the article “First WHO Global Clinical Trials Forum” published in “WHO” on 29th November 2023
Why in the News?
The First WHO Global Clinical Trials Forum was recently conducted. The objective of the forum was to develop a joint vision on strengthening clinical research capabilities.
What are Clinical Trials?
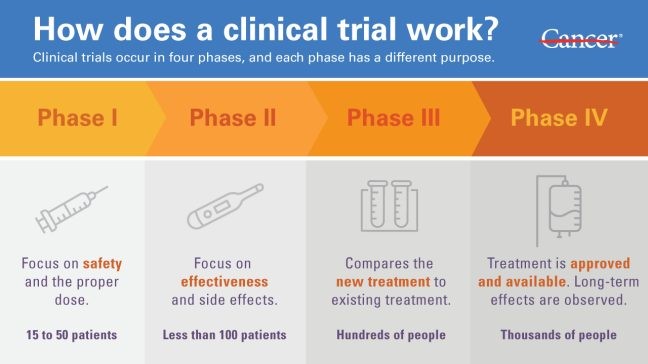
Clinical trials are a type of research that studies new tests and treatments and evaluates their effects on human health outcomes.
These trials are an essential component of the drug development process and are necessary to determine the benefits and risks of new treatments.
How are Clinical Trials regulated in India?
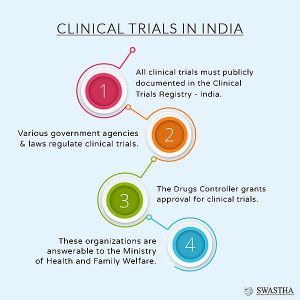
1) In India, the Central Drug Standard Control Organisation (CDSCO) regulates clinical trials under Drugs and Cosmetics Act, 1940.
2) Clinical Trials Registry – India (CTRI):
| Specifications | Details |
| Established in | 2007 |
| Hosted at | Indian Council of Medical Research (ICMR)’s National Institute of Medical Statistics. |
| Purpose | It is a free, online public-record system to register clinical trials being conducted in India. |
| Mandatory to register | It is mandatory to register for every clinical trial at CTRI before commencing as per the Drugs Controller General (India) (DCGI). |
3) New Drugs and Clinical Trial Rules: These rules were notified in 2019 with the aim to promote clinical research in the country.
– In 2023, an amendment was made to the rules to allow the researchers to utilize non-animal and human-relevant, including technologies like 3D organoids, organs-on-chip, and advanced computational methods, to test the safety and efficacy of new drugs.
UPSC Syllabus: Science and Technology




